Cell therapy involves the transfer of viable autologous or allogeneic cells into a patient’s body to replace diseased or damaged cells, modulate cell function, or assist in removing disease-causing or dysfunctional cells1. Cell therapies, encompassing both stem-cell and non-stem-cell therapies, have offered hope to patients and healthcare professionals with their potential to treat diseases once considered incurable2. Despite the promise of cell therapies, researchers and clinicians encounter significant hurdles in the research, development, and manufacturing stages. Here, we will explore recent advancements in cell therapy research, the opportunities and challenges associated with cell-based assays for cell therapy development, and suggest solutions for better outcomes.
Recent advancements in cell therapy research
Cell therapy has recently witnessed a remarkable transformation owing to technological and methodological advances that have significantly expanded its potential. A noteworthy development has been Chimeric Antigen Receptor (CAR)-T cell therapies, which have shown promise in treating hematological malignancies, offering patients significant improvements in their health and clinical outcomes and the opportunity to go into complete remission from cancer2. However, CAR-T therapy comes with its share of challenges, including potentially adverse effects, limited availability of autologous T cells, and the high cost and time requirements for manufacturing, which have limited its widespread clinical application3. CAR-natural killer (NK) cell therapy has emerged as a potential alternative that is expected to be more well-tolerated and affordable. However, it faces limitations in proliferative capacity, impacting its anti-tumor efficacy4,5.
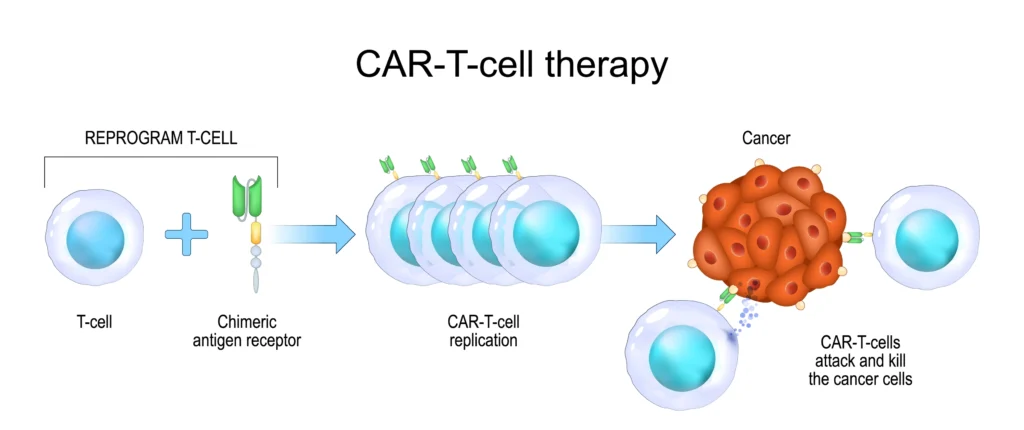
Despite significant advancements, cell therapy remains in its early stages, requiring extensive research efforts to unlock its full potential. To this end, precise, accurate, and efficient cell-based assays are crucial for improving research and manufacturing processes in cell therapy development.
Cell-based assays for cell therapy
Cell-based assays play a central role in the research and development of cell therapies. Given the complex nature of cell therapy products, assessing a broad spectrum of cell characteristics is essential to accurately predict their clinical effectiveness and safety. In line with this, the FDA requires potency assays to be included in cell therapy testing processes6. Often, measuring cell potency requires several complementary assays to be run in parallel to get a complete picture of the cell therapy’s characteristics and overcome the potential limitations of a single biological assay.
Some of the most commonly used cell-based assays relevant for cell potency testing include:
- Flow Cytometry – Often used to quantify viral transduction efficiency, protein expression, or other features associated with cell phenotype and function7.
- High-Throughput Imaging – Imaging-based assays can also monitor viral transduction and characterize cell phenotypes in response to treatments8.
- Metabolism Assays – Metabolism assays, such as seahorse assays, enable researchers to accurately monitor glycolytic and oxidative metabolism by measuring oxygen consumption in response to treatments and stimuli9.
- Sequencing – Single-cell sequencing technologies facilitate the high-resolution profiling of gene expression, interactions, immunity, and cellular heterogeneity10.
- Xenograft Models – Patient-derived xenograft models are used to study human tumor cell growth in vivo by implanting human tumor cells into immunocompromised or humanized mice11.
- Killing Assays – Killing or cytotoxicity assays are sensitive and specific means of measuring immune-cell-mediated killing of target tumor cells and determining cell therapy potency12.
- Growth Assays – Growth assays are essential for evaluating cell therapies’ proliferative and functional capabilities. Commonly used growth assays include MTT/MTS, CCK-8, and flow cytometry13.
- Proteomics – Proteomics profiling is a valuable and sensitive tool for characterizing cell therapy products and the molecular changes induced in target cell populations14.
The challenge of cell viability
Despite their importance for cell therapy research and process development, cell-based assays are not without challenges. The reliability of cell-based assay outcomes is determined by the quality of the starting material, which highlights the critical importance of ensuring only the highest quality cells are input.
Ensuring high-quality starting material involves conducting accurate, precise cell counts and viability monitoring15. The presence of dead or dying cells can lead to inaccurate cell counts, meaning that assays may be performed on an insufficient quantity of viable cells. Moreover, dead or dying cells and associated cellular debris and secreted factors can influence an assay’s reactivity and generate misleading signals, compromising the integrity of the results16,17. This can occur through the elevation of background noise, unintentional cellular activation, and transcriptional changes, among other artifacts, which can detract from the assay’s ability to accurately reflect the therapeutic potential and introduce variability in the results.
Not only can this lead to samples being wasted and readouts being inaccurate, but it can also lead to researchers drawing the wrong conclusions and cutting a potentially valuable therapy from development due to suspected potency issues when the real problem was with the viability of the test sample. Conversely, it can lead to higher doses being used in patients, which can be problematic in terms of manufacturing and adverse effects.
Consequently, cell-based assay manufacturers typically recommend maintaining a minimum viability of 70-90% in the starting material. This ensures unbiased outcomes in high-resolution assays, such as single-cell sequencing and functional metabolic phenotyping, where precision is paramount. However, guaranteeing that cell samples not only attain this viability standard but also do not contain debris or secreted factors can be challenging for researchers and excludes certain techniques such as beads, bubbles, and FACS, which generally do not have the capability to remove debris.
The solution: Levitation Technology for enhanced results
Levitation TechnologyTM presents an innovative and highly efficient alternative to conventional cell separation methods, significantly enhancing the accuracy and reliability of cell-based assays and the associated data. This technology uses a paramagnetic solution within a magnetic field to levitate viable cells away from dead or dying cells without the need for labels. This method not only simplifies the cell separation process to three straightforward steps, achievable in 20 minutes, but it also ensures high cell viability and yield, crucial for accurate and reliable cell-based assays. Unlike traditional methods involving harsh treatments that impact cell viability and integrity, Levitation Technology offers a label-free, gentle approach to cell enrichment, minimizing cell stress and maintaining integrity.
Conclusions and future perspectives
Cell therapy represents a transformative treatment that can potentially cure previously incurable diseases. Despite its potential, the field faces research, development, and manufacturing challenges requiring further advancements. Cell-based assays are central to navigating these challenges, offering insights into cell therapies’ potency, efficacy, and safety. Recent technological advancements, like LevitasBioⓇ’s Levitation Technology, further enhance the precision and reliability of these assays by enabling a simple cell enrichment process to obtain the highest quality cells to be used as starting material in these cell-based assays. To learn more about how the LevitasBio LeviCell® platform can support your cell potency assays, get in touch with one of our expert team members today!
References
6. Guidance for Industry Potency Tests for Cellular and Gene Therapy Products.
LeviCell systems and associated products are for Research Use Only, not for use in diagnostic procedures.